Aviation fuels must remain chemically stable during storage and inside the fuel tank when the aircraft is flying. However, the oxidation of the hydrocarbons in the fuel produces sticky gums, acids and insoluble residues, especially when the fuel comes into contact with warmer parts such as the fuel injection system. These gummy substances reduce fuel performance, increase emissions, and, ultimately, lead to engine malfunction.
So, to reduce free radical reactions, prevent or delay oxidation and maintain chemical stability of aviation fuels, antioxidants are added. One commonly used antioxidant is Topanol A, a phenol with a butyl group and two methyl groups.
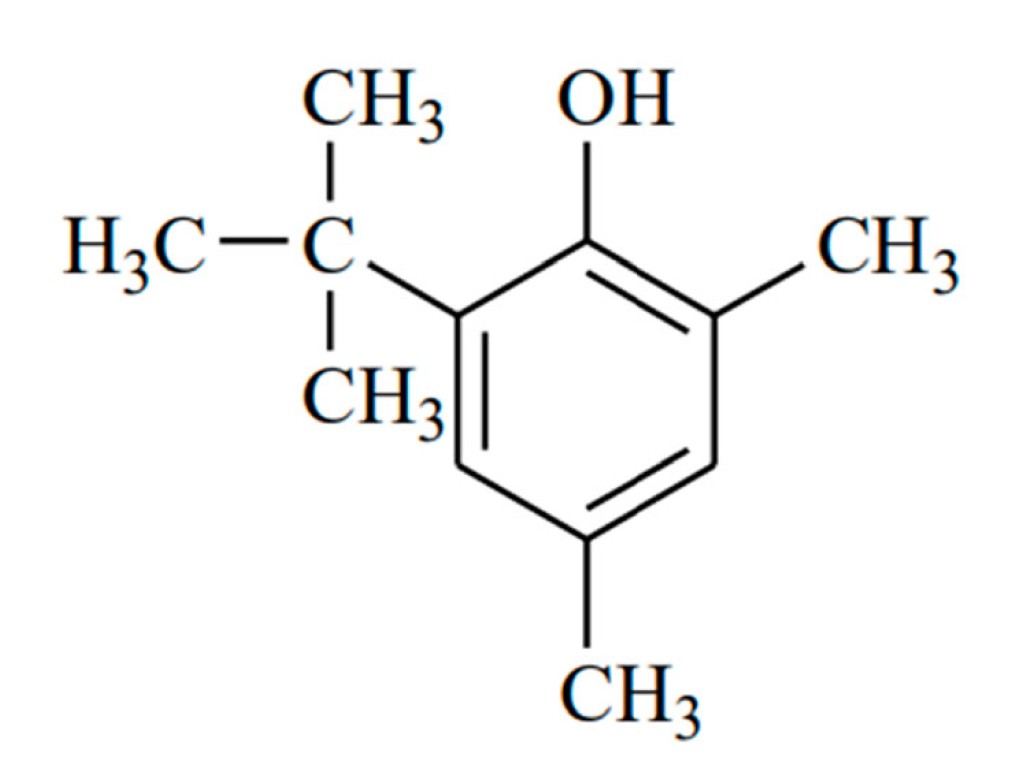
Image: structure of 2,6-di-tert-butylmethylphenol or tropanol A
Though the protective role of this chemical in aviation fuel is well recognised, the exact molecular mechanism is not known. What exactly helps this compound prevent oxidation in aviation fuels?
Experimental techniques cannot provide insights into fast electron or proton movements inside molecules. So, researchers at the M G University, Kottayam and IIT Madras used theoretical methods to tackle the problem.
First they used Vconf, a software programme, to draw the various possible configurations of tropanol A, as well as its ionic and radical forms. The software helped them identify the most stable configurations – those with the lowest Gibbs free energy.
Then they used the Multiwfn software package to visualise the electron density in frontier orbitals. The software helped them understand the spatial arrangement of unpaired electrons indicating regions with higher concentrations of electrons with up or down spin.
To solve the quantum equations computationally, they used the Minnesota functionals along with the various basis sets which can tackle even the problems posed by relatively heavier atoms. Thus, they could determine the structure and energetics as well as the approximate molecular orbitals of topanol A, its ions and radicals.
Now the remaining problem was to understand how the molecule works within the aviation fuel.
To represent aviation fuel, they used the structures of cyclohexane and n-octane. To understand how topanol A behaves when dissolved in cyclohexane and n-octane, the researchers used the polarisable continuum model in the Gaussian 16 software package and compared the results when topanol A is in gaseous form and is not dissolved.
In the gaseous phase, very small amounts of energy were needed to remove a hydrogen atom from the phenolic group. So the hydrogen atom transfer was the most favourable.
In non-polar solvents, however, the first reaction happens with the loss of a proton and then by the transfer of an electron from the phenolic group.
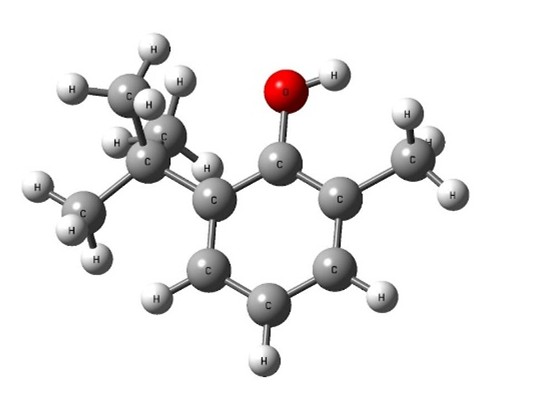
The oxygen atom of the hydroxyl group was the key reactive centre.
Image: ball and stick model of tropanol A
showing oxygen in red
The distribution of charges across the aromatic ring stabilised the resulting radical intermediate.
The electronic spectra showed a wide energy gap between the highest and lowest orbitals. This energy gap was the reason for the molecule’s high stability and antioxidant efficiency. Thus, the researchers established that fast hydrogen donation and charge redistribution are the factors that determine the antioxidant property of 2,4-dimethyl-6-tert-butylphenol in aviation fuels.
The research demonstrates how computational chemistry can help understand the mechanisms of chemical reactions. The day is not far when we use such techniques to develop new derivatives of 2,4-dimethyl-6-tert-butylphenol to enhance its antioxidant activity in aviation fuel or to even design of better fuel additives.
Molecular Physics, 123(4): 2024
DOI: 10.1080/00268976.2024.2393435
Reported by Ashtosh Gupta
Udai Pratap Autonomous College, Varanasi
This report was written during a workshop on science writing for science faculty with the aim of improving skills for writing better quality scientific papers, reviews and project proposals. Writing such reports helped participants understand the need to vary the style of writing depending on the purpose. The workshop was organised by scienceandmediaworkshops. Details of such workshops can be seen in this link.
The next workshop on science writing, starting on November 29, 2025, will focus on building the skills of scientists and faculty in agriculture, forestry, animal husbandry and fisheries. Here is the link to the announcement.
Leave a comment